Mission
Perform innovative, cutting-edge primary care research that improves the education, healthcare, and community outreach we deliver.
Vision
Develop the discoveries that enhance human health & well-being.
Strategic Priorities
Increase research funding and scholarly output and integrate DFMCH research discoveries into education curriculum, clinical practice, and community outreach.
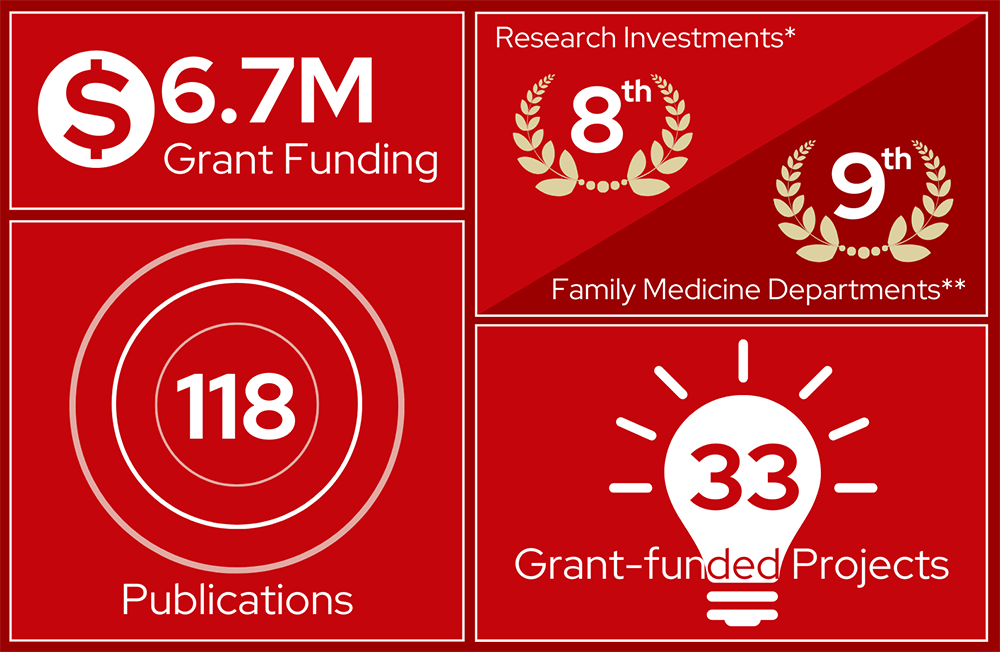
Reflects data from the FY23 annual report.
* UW-Madison’s ranking by the National Science Foundation
** 2023 US News and World Report specialty rankings
